燃烧a g乙醇(液态),生成二氧化碳气体和27g液态水,放出的热量为Q kJ,则表示乙醇燃烧热的热化学方程式书写正确的是
| A.C2H5OH(1)+3O2(g)=2CO2(g)+3H2O(1);△H = -QkJ/mol |
| B.C2H5OH(1)+3O2(g)=2CO2(g)+3H2O(1);△H =" -Q/2" kJ/mol |
| C.1/2 C2H5OH(1)+3/2O2(g)=CO2(g)+3/2H2O(1);△H = -QkJ/mol |
| D.C2H5OH(1)+3O2(g)=2CO2(g)+3H2O(1);△H =" -2Q" kJ/mol |
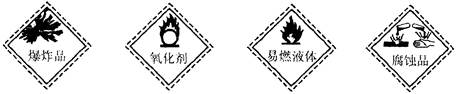

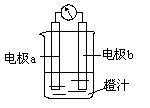
 为锌片时,导线中会产生电流
为锌片时,导线中会产生电流
 粤公网安备 44130202000953号
粤公网安备 44130202000953号