已知在发射卫星时可用肼(N2H4)为燃料、二氧化氮作氧化剂,这两者反应生成氮气和水蒸气。又知:①N2(g)+2O2(g)=2NO2(g)ΔH=+67.7kJ/mol
②N2H4(g)+O2(g)=N2(g)+2H2O(g) ΔH=-534kJ/mol
则肼与NO2反应的热化学方程式为
A.N2H4g)+NO2(g)=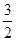 N2(g)+2H2O(g)ΔH=+567.85kJ/mol N2(g)+2H2O(g)ΔH=+567.85kJ/mol |
B.N2H4(g)+NO2(g)=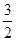 N2(g)+2H2O(g)ΔH=-567.85kJ/mol N2(g)+2H2O(g)ΔH=-567.85kJ/mol |
C.N2H4(g)+NO2(g)=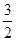 N2(g)+2H2O(l)ΔH=+567.85kJ/mol N2(g)+2H2O(l)ΔH=+567.85kJ/mol |
D.N2H4(g)+NO2(g)=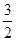 N2(g)+2H2O(l)ΔH=-567.85kJ/mol N2(g)+2H2O(l)ΔH=-567.85kJ/mol |

 粤公网安备 44130202000953号
粤公网安备 44130202000953号