N2H4是一种高效清洁的火箭燃料,0.25 mol N2H4(g)完全燃烧生成N2和气态水放出133.5kJ热量,则下列热化学方程式中正确的是
A.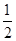 N2H4(g)+ N2H4(g)+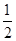 O2(g) O2(g) 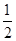 N2(g)+H2O(g)△H="+267" kJ·mol-1 N2(g)+H2O(g)△H="+267" kJ·mol-1 |
B.N2H4(g)+O2(g) N2(g)+2H2O(g)△H=-133.5 kJ·mol-1 N2(g)+2H2O(g)△H=-133.5 kJ·mol-1 |
C.N2H4(g)+O2(g) N2(g)+2H2O(l)△H=-534 kJ·mol-1 N2(g)+2H2O(l)△H=-534 kJ·mol-1 |
D.N2H4(g)+O2(g) N2(g)+2H2O(g)△H=-534 kJ·mol-1 N2(g)+2H2O(g)△H=-534 kJ·mol-1 |
 HBr+ HBrO 加入 AgNO3溶液后,溶液颜色变浅
HBr+ HBrO 加入 AgNO3溶液后,溶液颜色变浅 2C(g)+D(g) 已达平衡状态的是()
2C(g)+D(g) 已达平衡状态的是()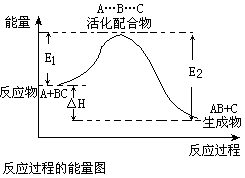 ,下列说法错误的是()
,下列说法错误的是() 粤公网安备 44130202000953号
粤公网安备 44130202000953号