25℃、101 kPa下,碳、氢气、甲烷和葡萄糖的燃烧热依次是393.5 kJ/mol、285.8 kJ/mol
890.3 kJ/mol、2800 kJ/mol,则下列热化学方程式正确的是
A.C(s)+ O2(g)=CO(g) △H= ―393.5 kJ/mol O2(g)=CO(g) △H= ―393.5 kJ/mol |
| B.2H2(g)+O2(g)=2H2O(l) △H= +571.6 kJ/mol |
| C.CH4(g)+2O2(g)=CO2(g)+2H2O(g) △H= ―890.3 kJ/mol |
| D.C6H12O6(s) +6O2(g)=6CO2(g)+6H2O(l) △H=" ―2800" kJ/mol |


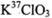 与浓盐酸(HCl)在加热时生成Cl2:
与浓盐酸(HCl)在加热时生成Cl2: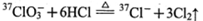
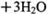
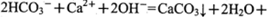
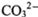
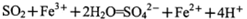
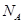 表示阿伏加德罗常数的值。下列叙述中正确的是
表示阿伏加德罗常数的值。下列叙述中正确的是 粤公网安备 44130202000953号
粤公网安备 44130202000953号