在25 ℃、101 kPa下,1 g甲醇燃烧生成CO2和液态水时放热22.68 kJ,下列热化学方程式正确的是
A.CH3OH(l)+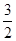 O2(g)===CO2(g)+2H2O(l)ΔH =+725.8 kJ/mol O2(g)===CO2(g)+2H2O(l)ΔH =+725.8 kJ/mol |
| B.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l)ΔH =-1452 kJ/mol |
| C.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O (l)ΔH =-725.8 kJ/mol |
| D.2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l)ΔH =+1452 kJ/mol |
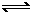 xC(g)ΔH<0
xC(g)ΔH<0
 的说法正确的是 ( )
的说法正确的是 ( ) 的结构式:
的结构式: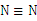
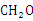


 2HI(g) ;△H<0,并达到平衡,HI的体积分数ω(HI)随时间变化如图(II)所示。若改变反应条件,ω(HI)的变化曲线如图所示,则改变的条件可能是 ( )
2HI(g) ;△H<0,并达到平衡,HI的体积分数ω(HI)随时间变化如图(II)所示。若改变反应条件,ω(HI)的变化曲线如图所示,则改变的条件可能是 ( )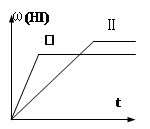
 粤公网安备 44130202000953号
粤公网安备 44130202000953号