已知:①CH3OH(g)+3/2O2(g) = CO2(g) + 2H2O(g) △H =" a" kJ·mol- 1
②CH4(g) + 2O2(g) = CO2(g) + 2H2O(g) △H =" b" kJ·mol- 1 则下列叙述正确的是
| A.C—H键键长小于H—H键 |
| B.甲烷的燃烧热为b kJ·mol -1 |
| C.2CH3OH(g)=2CH4(g)+O2(g)△H=2(a—b) kJ·mol- 1 |
| D.当甲醇和甲烷物质的量之比为l:2时,其完全燃烧生成CO2和H2O(g)时,放出的热量为c kJ,则 |
该混合物中甲醇的物质的量为c/(a+2b)mol
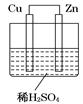
 向Cu极移动
向Cu极移动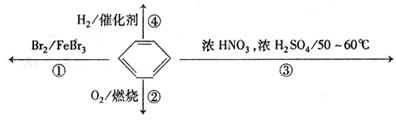
 粤公网安备 44130202000953号
粤公网安备 44130202000953号