已知下列反应的反应热:
(1)CH3COOH(l)+2O2(g) 2CO2(g)+2H2O(l) ΔH1=-870.3 kJ·mol-1
2CO2(g)+2H2O(l) ΔH1=-870.3 kJ·mol-1
(2)C(s)+O2(g) CO2(g) ΔH2=-393.5 kJ·mol-1
CO2(g) ΔH2=-393.5 kJ·mol-1
(3)H2(g)+O2(g) H2O(l) ΔH3=-285.8 kJ·mol-1
H2O(l) ΔH3=-285.8 kJ·mol-1
则下列反应的反应热为( )
2C(s)+2H2(g)+O2(g) CH3COOH(l)
CH3COOH(l)
| A.ΔH=+488.3 kJ·mol-1 |
| B.ΔH=-244.15 kJ·mol-1 |
| C.ΔH=-977.6 kJ·mol-1 |
| D.ΔH=-488.3 kJ·mol-1 |
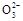 +7H2O,下列叙述正确的是( )。
+7H2O,下列叙述正确的是( )。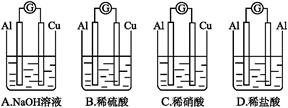
 只向PbO2处移动
只向PbO2处移动 粤公网安备 44130202000953号
粤公网安备 44130202000953号