在101kPa 25℃时,1.0g乙烷气体完全燃烧生成液态水时放出热量52.0kJ,则乙烷燃烧的热化学方程式为
A.C2H6(g) +  O2(g)=2CO2(g) +3H2O(l)△H =-1560kJ·mol-1 O2(g)=2CO2(g) +3H2O(l)△H =-1560kJ·mol-1 |
| B.2C2H6(g) + 7O2(g)=4CO2(g) +6H2O(g)△H =-1560kJ·mol-1 |
| C.2C2H6(g) + 7O2(g)=4CO2(g) +6H2O(l)△H =+3120 kJ·mol-1 |
D.C2H6(g) + O2(g)=2CO2(g) +3H2O(l)△H =-52.0kJ·mol-1 O2(g)=2CO2(g) +3H2O(l)△H =-52.0kJ·mol-1 |
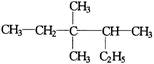 的正确命名为
的正确命名为
 粤公网安备 44130202000953号
粤公网安备 44130202000953号