N2H4是一种高效清洁的火箭燃料.0.25mol N2H4(g)完全燃烧生成氮气和气态水时,放出133.5kJ热量.则下列热化学方程中正确的是( )
A.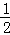 N2H4(g)+ N2H4(g)+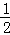 O2(g)= O2(g)=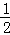 N2(g)+H2O(l)△H=+257kJ•mol﹣1 N2(g)+H2O(l)△H=+257kJ•mol﹣1 |
| B.N2H4(g)+O2(g)=N2(g)+2H2O(l)△H=﹣133.5kJ•mol﹣1 |
| C.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H=+534kJ•mol﹣1 |
| D.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H=﹣534kJ•mol﹣1 |
 粤公网安备 44130202000953号
粤公网安备 44130202000953号