25℃、101 kPa下,碳、氢气、甲烷和葡萄糖的燃烧热依次是393.5 kJ/mol、285.8 kJ/mol、890.3 kJ/mol、2800 kJ/mol,则下列热化学方程式正确的是
| A.C(s)+1/2O2(g)=CO(g) △H =" -" 393.5 kJ/mol |
| B.2H2(g)+O2(g)=2H2O(l) △H =" +" 571.6 kJ/mol |
| C.CH4(g)+2O2(g)=CO2(g)+2H2O(g) △H =" -" 890.3 kJ/mol |
| D.C6H12O6(s) +6O2(g)=6CO2(g)+6H2O(l) △H =" -" 2800 kJ/mol |
 2Z(g);ΔH<0。如下图表示该反应的速率(v)随时间(t)变化的关系,t2、t3、t5时刻外界条件有所改变,但都没有改变各物质的初始加入量。下列说法中不正确的是( )
2Z(g);ΔH<0。如下图表示该反应的速率(v)随时间(t)变化的关系,t2、t3、t5时刻外界条件有所改变,但都没有改变各物质的初始加入量。下列说法中不正确的是( )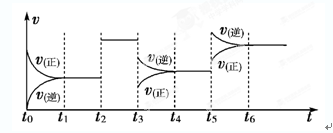

 粤公网安备 44130202000953号
粤公网安备 44130202000953号