沼气是一种能源,它的主要成分是CH4。0.5 mol CH4完全燃烧生成CO2和液态水时放出445 kJ的热量,则下列热化学方程式中正确的是( )
A.CH4(g)+2O2(g)==CO2(g)+2H2O(1) △H=" —890" kJ/mol
CH4(g)+2O2(g)==CO2(g)+2H2O(l) △H=" +890" kJ/mol
C.2CH4(g)+4O2(g)==2CO2(g)+4H2O(1) △H=" +1780" kJ/mol
D.1/2 CH4(g)+O2(g)="=1/2" CO2(g)+H2O(1) △H=" —890" kJ/mol
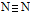 的键能为942 KJ/mol。则下列说法不正确的是
的键能为942 KJ/mol。则下列说法不正确的是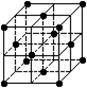
 粤公网安备 44130202000953号
粤公网安备 44130202000953号