下列热化学方程式中,△H能表示对应物质的标准燃烧热的是
A.CO(g)+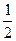 O2(g)==CO2(g)△H =-283.0 kJ•mol—1 O2(g)==CO2(g)△H =-283.0 kJ•mol—1 |
B.C(s)+ 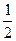 O2(g)="=CO(g)" △H = -110.5 kJ•mol—1 O2(g)="=CO(g)" △H = -110.5 kJ•mol—1 |
C.H2(g)+ 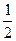 O2(g)==H2O(g)△H = -241.8 kJ•mol—1 O2(g)==H2O(g)△H = -241.8 kJ•mol—1 |
| D.2C8H18O(l)+25O2(g)==16CO2(g)+18H2O(l)△H =-11828.0 kJ•mol—1 |
下列热化学方程式中,△H能表示对应物质的标准燃烧热的是
A.CO(g)+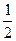 O2(g)==CO2(g)△H =-283.0 kJ•mol—1 O2(g)==CO2(g)△H =-283.0 kJ•mol—1 |
B.C(s)+ 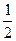 O2(g)="=CO(g)" △H = -110.5 kJ•mol—1 O2(g)="=CO(g)" △H = -110.5 kJ•mol—1 |
C.H2(g)+ 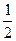 O2(g)==H2O(g)△H = -241.8 kJ•mol—1 O2(g)==H2O(g)△H = -241.8 kJ•mol—1 |
| D.2C8H18O(l)+25O2(g)==16CO2(g)+18H2O(l)△H =-11828.0 kJ•mol—1 |