常温常压下,充分燃烧一定量的甲烷放出热量222.575KJ,经测定完全吸收生成的二氧化碳需消耗1mol/L的NaOH溶液250mL,恰好生成正盐。则此条件下甲烷的燃烧热热化学方程式 ( )
| A.CH4(g)+2O2(g)=CO2(g)+2H2O(g);△H="-890.3" kJ/mol |
| B.1/2CH4(g)+O2(g)=1/2CO2(g)+H2O(l);△H="-222.575" kJ/mol |
| C.1/2CH4(g)+O2(g)=1/2CO2(g)+H2O(g);△H="-445.15" kJ/mol |
| D.CH4(g)+2O2(g)=CO2(g)+2H2O(l);△H="-890.3" kJ/mol |
 C(g)+D(g)。当下列物理量不再发生变化时,能表明该反应已达到平衡状态的是( )
C(g)+D(g)。当下列物理量不再发生变化时,能表明该反应已达到平衡状态的是( ) 2SO3(g),下列措施能使反应物中活化分子百分数、化学反应速率和化学平衡常数都变化的是( )
2SO3(g),下列措施能使反应物中活化分子百分数、化学反应速率和化学平衡常数都变化的是( )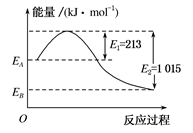

 O2(g)===H2O(l)ΔH=-285.8 kJ·mol-1
O2(g)===H2O(l)ΔH=-285.8 kJ·mol-1 粤公网安备 44130202000953号
粤公网安备 44130202000953号