肼(N2H4)是火箭发动机的燃料,它与N2O4反应时,N2O4为氧化剂,生成氮气和水蒸气。已知:N2(g)+2O2(g)===N2O4(g) ΔH=+8.7 kJ/mol,N2H4(g)+O2(g)===N2(g)+2H2O(g) ΔH=-534.0 kJ/mol,下列表示肼跟N2O4反应的热化学方程式,正确的是
| A.2N2H4(g)+N2O4(g)===3N2(g)+4H2O(g)ΔH=-542.7 kJ/mol |
| B.2N2H4(g)+N2O4(g)===3N2(g)+4H2O(g) ΔH=-1 059.3 kJ/mol |
| C.2N2H4(g)+N2O4(g)===3N2(g)+4H2O(g)ΔH=-1 076.7 kJ/mol |
D.N2H4(g)+ N2O4(g)=== N2O4(g)===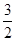 N2(g)+2H2O(g)ΔH=-1 076.7 kJ/mol N2(g)+2H2O(g)ΔH=-1 076.7 kJ/mol |
 粤公网安备 44130202000953号
粤公网安备 44130202000953号