、N2H2是一种高效清洁的火箭燃料。0.25 mol N2H2(g)完全燃烧生成氮气和气态水时,放出133.5 kJ热量。则下列热化学方程式正确的是
| A.N2H2(g)+O2(g)=N2(g)+2H2O(g);ΔH="+133.5" kJ·mol-1 |
| B.N2H2(g)+O2(g)=N2(g)+2H2O(g);ΔH="-133.5" kJ·mol-1 |
| C.N2H2(g)+O2(g)=N2(g)+2H2O(g);ΔH="+534" kJ·mol-1 |
| D.N2H2(g)+O2(g)=N2(g)+2H2O(g);ΔH="-534" kJ·mol-1 |
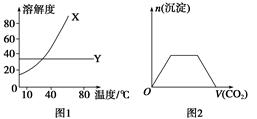
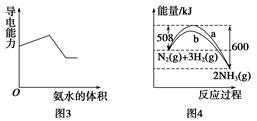

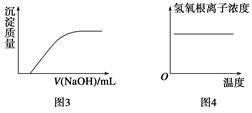
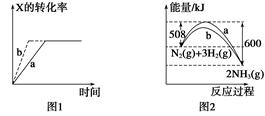
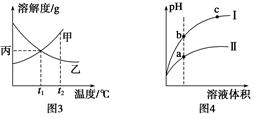
 3Z(g),b的压强一定比a大
3Z(g),b的压强一定比a大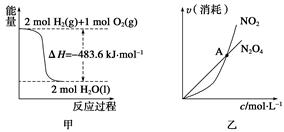
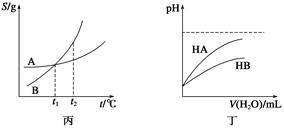
 N2O4(g)中,各物质的浓度与其消耗速率之间的关系,其中交点A对应的状态为化学平衡状态
N2O4(g)中,各物质的浓度与其消耗速率之间的关系,其中交点A对应的状态为化学平衡状态 粤公网安备 44130202000953号
粤公网安备 44130202000953号