在1.01KPa和298K条件下,2MolH2生成水蒸汽放出484kJ热量,下列热化学方程式正确的是
| A.2H2(g)+O2(g)=2H2O(l); △H=-484kJ/Mol |
B.H2O(g)= H2(g)+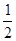 O2(g); △H=+242kJ/Mol O2(g); △H=+242kJ/Mol |
| C.2H2(g)+ O2(g)=2H2O(g); △H=-484kJ/Mol |
D.H2(g)+ 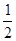 O2(g)= H2O(g); △H=+242kJ/Mol O2(g)= H2O(g); △H=+242kJ/Mol |
在1.01KPa和298K条件下,2MolH2生成水蒸汽放出484kJ热量,下列热化学方程式正确的是
| A.2H2(g)+O2(g)=2H2O(l); △H=-484kJ/Mol |
B.H2O(g)= H2(g)+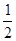 O2(g); △H=+242kJ/Mol O2(g); △H=+242kJ/Mol |
| C.2H2(g)+ O2(g)=2H2O(g); △H=-484kJ/Mol |
D.H2(g)+ 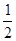 O2(g)= H2O(g); △H=+242kJ/Mol O2(g)= H2O(g); △H=+242kJ/Mol |