Fe2O3(s)+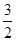 C(s)===
C(s)===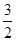 CO2(g)+2Fe(s) ΔH1=+234.1 kJ/mol;C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol;则2Fe(s)+
CO2(g)+2Fe(s) ΔH1=+234.1 kJ/mol;C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol;则2Fe(s)+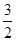 O2(g)===Fe2O3(s)的ΔH是
O2(g)===Fe2O3(s)的ΔH是
| A.-627.6 kJ/mol | B.-824.4 kJ/mol |
| C.-744.7 kJ/mol | D.-169.4 kJ/mol |
Fe2O3(s)+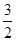 C(s)===
C(s)===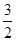 CO2(g)+2Fe(s) ΔH1=+234.1 kJ/mol;C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol;则2Fe(s)+
CO2(g)+2Fe(s) ΔH1=+234.1 kJ/mol;C(s)+O2(g)===CO2(g) ΔH2=-393.5 kJ/mol;则2Fe(s)+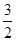 O2(g)===Fe2O3(s)的ΔH是
O2(g)===Fe2O3(s)的ΔH是
| A.-627.6 kJ/mol | B.-824.4 kJ/mol |
| C.-744.7 kJ/mol | D.-169.4 kJ/mol |