已知下列热化学反应方程式:
① Fe2O3(s)+3CO(g) = 2Fe(s)+3CO2(g) ΔH =-24.8 kJ/mol
② Fe2O3(s)+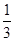 CO(g) =
CO(g) =  Fe3O4(s)+
Fe3O4(s)+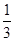 CO2(g) ΔH =-15.73 kJ/mol
CO2(g) ΔH =-15.73 kJ/mol
③ Fe3O4(s)+CO(g) =3FeO(s)+CO2(g) ΔH =+640.4 kJ/mol
则14 g CO气体还原足量FeO固体得到Fe固体和CO2气体时对应的ΔH约为
| A.-218 kJ/mol | B.-109 kJ/mol |
| C.+218 kJ/mol | D.+109 kJ/mol |


 ,氧化性:HClO>Cl2>Br2>Fe3+>I2。下列有关离子反应或离子方程式的叙述中,正确的是
,氧化性:HClO>Cl2>Br2>Fe3+>I2。下列有关离子反应或离子方程式的叙述中,正确的是 >
> >I2
>I2 粤公网安备 44130202000953号
粤公网安备 44130202000953号