分析下列反应在任何温度下均能自发进行的是
| A.2N2(g)+O2(g)===2N2O(g) ΔH=+163 kJ·mol-1 |
B.Ag(s)+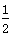 Cl2(g)===AgCl(s) ΔH=-127 kJ·mol-1 Cl2(g)===AgCl(s) ΔH=-127 kJ·mol-1 |
C.HgO(s)===Hg(l)+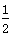 O2(g) ΔH=+91 kJ·mol-1 O2(g) ΔH=+91 kJ·mol-1 |
D.H2O2(l)=== 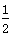 O2(g)+H2O(l) ΔH=-98 kJ·mol-1 O2(g)+H2O(l) ΔH=-98 kJ·mol-1 |
分析下列反应在任何温度下均能自发进行的是
| A.2N2(g)+O2(g)===2N2O(g) ΔH=+163 kJ·mol-1 |
B.Ag(s)+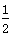 Cl2(g)===AgCl(s) ΔH=-127 kJ·mol-1 Cl2(g)===AgCl(s) ΔH=-127 kJ·mol-1 |
C.HgO(s)===Hg(l)+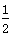 O2(g) ΔH=+91 kJ·mol-1 O2(g) ΔH=+91 kJ·mol-1 |
D.H2O2(l)=== 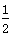 O2(g)+H2O(l) ΔH=-98 kJ·mol-1 O2(g)+H2O(l) ΔH=-98 kJ·mol-1 |