已知在1×105 Pa, 298 K条件下,2 mol氢气燃烧生成水蒸气放出484 kJ热量,下列热化学方程式正确的是
H2O ( g ) = H2 ( g ) + 1/2O2 ( g ) △H =" +242" kJ/mol
2H2 ( g ) + O2 ( g ) = 2H2O ( l ) △H = -484 kJ/mol
H2 ( g ) + 1/2O2 ( g ) = H2O (g ) △H =" +242" kJ/mol
2H2 ( g ) + O2 ( g ) = 2H2O ( g ) △H =" +484" kJ/mol
 H+(aq)+F-(aq)△H>0
H+(aq)+F-(aq)△H>0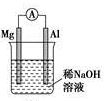 ②
② ③
③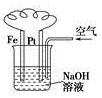 ④
④
 C(s)+H2O(g)△H>0的化学反应速率与实践的关系,图二表示的是可逆反应2NO2(g)
C(s)+H2O(g)△H>0的化学反应速率与实践的关系,图二表示的是可逆反应2NO2(g)
 粤公网安备 44130202000953号
粤公网安备 44130202000953号