已知下列反应的反应热:
(1)CH3COOH(l)+2O2(g) 2CO2(g)+2H2O(l) ΔH1="-870.3" kJ·mol-1
2CO2(g)+2H2O(l) ΔH1="-870.3" kJ·mol-1
(2)C(s)+O2(g) CO2(g) ΔH2="-393.5" kJ·mol-1
CO2(g) ΔH2="-393.5" kJ·mol-1
(3)H2(g)+O2(g) H2O(l) ΔH3="-285.8" kJ·mol-1
H2O(l) ΔH3="-285.8" kJ·mol-1
则下列反应的反应热为( )
2C(s)+2H2(g)+O2(g) CH3COOH(l)
CH3COOH(l)
| A.ΔH="+488.3" kJ·mol-1 | B.ΔH="-244.15" kJ·mol-1 |
| C.ΔH="-977.6" kJ·mol-1 | D.ΔH="-488.3" kJ·mol-1 |
 R(g)+2L,此反应符合下面图像,下列叙述正确的是()
R(g)+2L,此反应符合下面图像,下列叙述正确的是()
 pC(气), 达到平衡后, 若将混合气体的体积压缩到原来的 1/2, 当再次达到平衡时, C的浓度为原平衡时C的浓度的 1 .9 倍, 则下列叙述中正确的是()
pC(气), 达到平衡后, 若将混合气体的体积压缩到原来的 1/2, 当再次达到平衡时, C的浓度为原平衡时C的浓度的 1 .9 倍, 则下列叙述中正确的是() CO(g)+H2(g),当增加反应物物质的量时,平衡一定向正反应方向移动
CO(g)+H2(g),当增加反应物物质的量时,平衡一定向正反应方向移动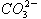
 粤公网安备 44130202000953号
粤公网安备 44130202000953号