2molCl2(g)与足量的H2(g)反应生成HCl(g),在298K时测得放出369.2kJ热量。则下列热化学方程式不正确的是
| A.H2(g)+ Cl2(g)= 2HCl(g)△H =" -" 369.2kJ·mol-1 |
| B.2H2(g)+2Cl2(g)= 4HCl(g)△H =" -" 369.2kJ·mol-1 |
| C.HCl(g)= 1/2H2(g)+ 1/2Cl2(g)△H =" +" 92.3kJ·mol-1 |
| D.1/2H2(g)+ 1/2Cl2(g)= HCl(g)△H =" -" 92.3kJ·mol-1 |
 2NH3(g)ΔH ="-92.4" kJ?mol-1,充分反应后放出的热量为
2NH3(g)ΔH ="-92.4" kJ?mol-1,充分反应后放出的热量为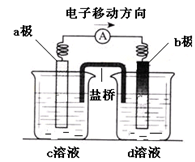
 粤公网安备 44130202000953号
粤公网安备 44130202000953号