下列热化学方程式及说法正确的是( )
| A.CH4(g)+ 2O2(g)=CO2(g)+2H2O(l) △H= —890KJ |
| B.丁烷的燃烧热是2878 kJ/mol,则: C4H10(g)+13/2O2(g)=4CO2(g)+5H2O(g)△H=" —2878kJ/mol" |
| C.2 mol氢气燃烧生成水蒸气放出484 kJ热量, 则:H2O(g)=H2(g)+1/2O2(g);△H="+242" kJ·mol-1 |
| D.CH3COOH(aq)+KOH(aq)=CH3COOK(aq)+H2O(l)△H=-akJ·mol-1,a就为中和热的值 |
 。下列说法正确的是( )
。下列说法正确的是( ) ,
,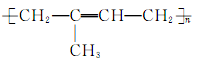 )
) )
) )
)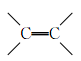 断裂,使其由线型结构变成体型结构)
断裂,使其由线型结构变成体型结构) 粤公网安备 44130202000953号
粤公网安备 44130202000953号