25 ℃,101 k Pa时,强酸与强碱的稀溶液发生中和反应的中和热为-57.3 kJ/mol,辛烷的燃烧热为-5518 kJ/mol。下列热化学方程式书写正确的是( )
A.2H+(aq) + (aq)+ (aq)+ (aq)+2 (aq)+2 (aq) = BaSO4(s)+2H (aq) = BaSO4(s)+2H O(l); O(l); H = H = 57.3 kJ/mol 57.3 kJ/mol |
B.KOH(aq)+ H H SO4(aq) = SO4(aq) =  K K SO4(aq)+H SO4(aq)+H O(l); O(l); H= H= 57.3kJ/mol 57.3kJ/mol |
C.C8H18(l)+  O O (g)=8CO (g)=8CO (g)+ 9H (g)+ 9H O(g); O(g); H= H= 5518 kJ/mol 5518 kJ/mol |
D.2C8H18(g)+25O (g)=16CO (g)=16CO (g)+18H (g)+18H O(1); O(1); H= H= 5518 kJ/mol 5518 kJ/mol |
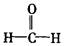 )分子中的四个原子是共平面的.下列分子中所有原子不可能同时存在同一平面上的是()
)分子中的四个原子是共平面的.下列分子中所有原子不可能同时存在同一平面上的是()

 粤公网安备 44130202000953号
粤公网安备 44130202000953号