对于热方程式说法正确的是:
A.中和热ΔH=-57.3 kJ·mol-1,, 则2H+(aq)+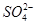 (aq)+Ba2+(aq)+2OH-(aq)===BaSO4(s)+2H2O(l) △H=-114.6 kJ·mol-1 (aq)+Ba2+(aq)+2OH-(aq)===BaSO4(s)+2H2O(l) △H=-114.6 kJ·mol-1 |
| B.将0.5 molN2和1.5 mol H2于密闭容器生成NH3(g),放热19.3 kJ,方程式为: N2(g)+3H2(g)  2NH3(g)△H="—38.6" kJ·mol—1 2NH3(g)△H="—38.6" kJ·mol—1 |
| C.标准状况:H2(g)+F2(g) ==="2HF(g)" △H=-270kJ/mol, |
| D.氨氧化:4NH3(g)+5O2(g) ===4NO(g)+6H2O(g)△H=-1025 kJ/mol |
 粤公网安备 44130202000953号
粤公网安备 44130202000953号