常温下,下列各溶液的叙述中正确的是
| A.pH=7的醋酸钠和醋酸混合液中:c(Na+)=c(CH3COO-) |
| B.0.1mol/L的醋酸的pH=a,0.01mol/L的醋酸的pH=b,则a+1>b |
| C.0.1mol/L的醋酸钠溶液20mL与0.1mol/L盐酸10mL混合后溶液显酸性 c (CH3COO-)>c (Cl-)>c (H+)>c (CH3COOH) |
| D.已知酸性HF>CH3COOH,pH相等的NaF与CH3COOK溶液中, |
[c(Na+)-c(F一)]<[c(K+)-c(CH3COO一)]
 和H3O+,
和H3O+,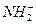 和OH-,N3-和O2-两两类似,据此判断下列反应不正确的是()
和OH-,N3-和O2-两两类似,据此判断下列反应不正确的是()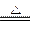 Mg3N2+3NH3↑
Mg3N2+3NH3↑ 粤公网安备 44130202000953号
粤公网安备 44130202000953号