已知在25℃、101kPa下,1gC8H18(辛烷)燃烧生成二氧化碳和液态水时放出48.40kJ 热量。表示上述反应的热化学方程式正确的是
| A.C8H18(l)+25/2O2(g)=8CO2(g) +9H2O(g)△H=" -" 48.40kJ·mol -1 |
| B.C8H18(l)+25/2O2(g)=8CO2 (g) +9H2O(l)△H=" -" 5518kJ·mol -1 |
| C.C8H18(l)+25/2O2(g)=8CO2(g) + 9H2O(l) △H=" +" 5518kJ·mol -1 |
| D.C8H18(l)+25/2O2(g)=8CO2 (g)+ 9H2O(l)△H= - 48.40kJ·mol -1 |
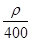 mol·L-1
mol·L-1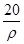 mol·L-1
mol·L-1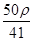 mol·L-1
mol·L-1 mol·L-1
mol·L-1 粤公网安备 44130202000953号
粤公网安备 44130202000953号