沼气是一种能源,它的主要成分是CH4。0.5 mol CH4完全燃烧生成CO2和液态水时放出445 kJ的热量,则下列热化学方程式中正确的是
| A.2CH4(g)+4O2(g)==2CO2(g)+4H2O(1)△H=" +1780" kJ/mol |
| B.CH4(g)+2O2(g)==CO2(g)+2H2O(l)△H=" +890" kJ/mol |
| C.CH4(g)+2O2(g)==CO2(g)+2H2O(1)△H= —890 kJ/mol |
D.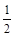 CH4(g)+O2(g)== CH4(g)+O2(g)==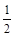 CO2(g)+H2O(1)△H= —890 kJ/mol CO2(g)+H2O(1)△H= —890 kJ/mol |
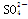 、Ca2+、Mg2+、Fe3+等杂质,加入的药品顺序为:Na2CO3溶液→NaOH溶液→BaCl2溶液→过滤后加盐酸
、Ca2+、Mg2+、Fe3+等杂质,加入的药品顺序为:Na2CO3溶液→NaOH溶液→BaCl2溶液→过滤后加盐酸
 粤公网安备 44130202000953号
粤公网安备 44130202000953号