下列有关电解质溶液中微粒的物质的量浓度关系正确的是( )
A.在0.1 mol/L NaHCO3溶液中:c(Na+) ﹥c(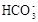 ) ﹥ c( ) ﹥ c(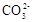 ) ﹥ c(H2CO3) ) ﹥ c(H2CO3) |
B.在0.1 mol/L Na2CO3溶液中:c(OH-) – c(H+) = c(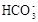 ) + c(H2CO3) ) + c(H2CO3) |
| C.向0.2 mol/L NaHCO3溶液中加入等体积0.1 mol/L NaOH溶液: c( 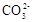 ) ﹥ c( ) ﹥ c(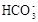 ) ﹥ c(OH-) ﹥ c(H+) ) ﹥ c(OH-) ﹥ c(H+) |
| D.向 0.1 mol/L的CH3COONa溶液中加入等体积0.1 mol/L CH3COOH溶液: |
2c(H+) – c(CH3COO-) = 2c(OH-) – c(CH3COOH)



 粤公网安备 44130202000953号
粤公网安备 44130202000953号