1g碳与适量水蒸气反应生成CO和H2,需吸收10.94kJ热量,此反应的热化学方程式为( )
A.C + H2O ="=" CO + H2△H= +131.3 kJ·m ol-1 ol-1 |
| B.C(s)+ H2O(g) ="=" CO(g) + H2(g)△H= +10.94 kJ·mol-1 |
| C.C(s)+ H2O(l) ="=" CO(g) + H2(g)△H= +131.3 kJ·mol-1 |
| D.C(s)+ H2O(g) ="=" CO(g) + H2(g)△H= +131.3 kJ·mol-1 |
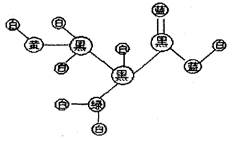
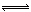 2NH3的平衡体系中,保持容器的体积和温度不变,充入氩气以增大压强,此时下列叙述正确的是
2NH3的平衡体系中,保持容器的体积和温度不变,充入氩气以增大压强,此时下列叙述正确的是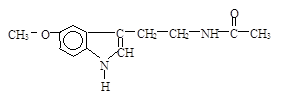
 O2(g) = H2O(l);△H =-285.8kJ/mol
O2(g) = H2O(l);△H =-285.8kJ/mol 粤公网安备 44130202000953号
粤公网安备 44130202000953号