已知热化学方程式:SO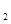 (g)+
(g)+ 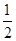 O
O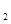 (g)
(g)  SO
SO (g) △H=-98.32kJ·mol
(g) △H=-98.32kJ·mol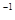 ,在容器中充人2molSO
,在容器中充人2molSO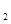 和1molO
和1molO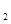 充分反应,最终放出的热量为 ( )
充分反应,最终放出的热量为 ( )
| A.196.64kJ | B.196.64 kJ·mol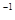 |
C.<196.64kJ | D.>196.64kJ |
已知热化学方程式:SO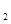 (g)+
(g)+ 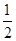 O
O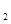 (g)
(g)  SO
SO (g) △H=-98.32kJ·mol
(g) △H=-98.32kJ·mol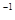 ,在容器中充人2molSO
,在容器中充人2molSO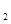 和1molO
和1molO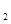 充分反应,最终放出的热量为 ( )
充分反应,最终放出的热量为 ( )
| A.196.64kJ | B.196.64 kJ·mol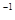 |
C.<196.64kJ | D.>196.64kJ |