已知1g火箭燃料肼(N2H4)气体燃烧生成N2和H2O(g)时,放出16.7kJ的热量,则该反应的热化学方程式正确的是
A.N2H4+O 2=N2+2H2O△H=-534.4kJ/mol 2=N2+2H2O△H=-534.4kJ/mol |
| B.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H =-534.4kJ/mol |
| C.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H = +534.4kJ/mol |
D.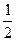 N2H4(g)+ N2H4(g)+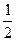 O2(g)= O2(g)=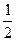 N2(g)+H2O(g)△H =-16.7kJ/mol N2(g)+H2O(g)△H =-16.7kJ/mol |
已知1g火箭燃料肼(N2H4)气体燃烧生成N2和H2O(g)时,放出16.7kJ的热量,则该反应的热化学方程式正确的是
A.N2H4+O 2=N2+2H2O△H=-534.4kJ/mol 2=N2+2H2O△H=-534.4kJ/mol |
| B.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H =-534.4kJ/mol |
| C.N2H4(g)+O2(g)=N2(g)+2H2O(g)△H = +534.4kJ/mol |
D.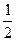 N2H4(g)+ N2H4(g)+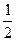 O2(g)= O2(g)=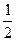 N2(g)+H2O(g)△H =-16.7kJ/mol N2(g)+H2O(g)△H =-16.7kJ/mol |