某工厂欲用2%的稀硫酸测定本厂排放的废水中氢氧化钾的含量(废水中的其他物质不与稀硫酸反应)。试计算:
(1)用40%的浓硫酸(密度为1.30 g/cm3)l0mL配制2%的稀硫酸,需加水(密度为1.0 g/cm3)多少毫升?
(2)向盛有20 g废水的锥形瓶中逐滴滴加2%的稀硫酸,至恰好完全反应。将实验所得数据绘制成右图所示图象。求废水中氢氧化钾的质量分数?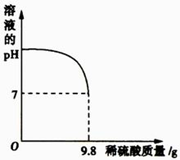
某工厂欲用2%的稀硫酸测定本厂排放的废水中氢氧化钾的含量(废水中的其他物质不与稀硫酸反应)。试计算:
(1)用40%的浓硫酸(密度为1.30 g/cm3)l0mL配制2%的稀硫酸,需加水(密度为1.0 g/cm3)多少毫升?
(2)向盛有20 g废水的锥形瓶中逐滴滴加2%的稀硫酸,至恰好完全反应。将实验所得数据绘制成右图所示图象。求废水中氢氧化钾的质量分数?