醋酸溶液中存在电离平衡:CH3 COOH H++CH3 COO-,下列叙述不正确的是 ( )
| A.CH3 COOH 溶液中离子浓度的关系满足:c(H+)= c(OH-)+c(CH3 COO-) |
B.0.1 mol ·L-1的CH3 COOH 溶液加水稀释,溶液 中c(OH-)减小 中c(OH-)减小 |
| C.CH3 COOH 溶液中加入少量CH3 COONa固体,平衡逆向移动 |
| D.常温下, pH=2的CH3 COOH 溶液与pH=12的NaOH 溶液等体积混合后,溶液的pH<7 |
 C (g)+D (g)达到平衡的标志是
C (g)+D (g)达到平衡的标志是
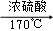 CH2=CH2↑+H2O)与溴水作用制取1,2﹣二溴乙烷的部分装置图,根据图示判断下列说法正确的是
CH2=CH2↑+H2O)与溴水作用制取1,2﹣二溴乙烷的部分装置图,根据图示判断下列说法正确的是
 粤公网安备 44130202000953号
粤公网安备 44130202000953号