下面均是正丁烷与氧气反应的热化学方程式(25℃,101kPa):
①C4H10(g)+13/2O2(g)=4CO2(g)+5H2O(l);ΔH=-2878kJ/mol
②C4H10(g)+13/2O2(g)=4CO2(g)+5H2O(g);ΔH=-2658kJ/mol
③C4H10(g)+9/2O2(g)=4CO(g)+5H2O(l);ΔH=-1746kJ/mol
④C4H10( g)+9/2O2(g)=4CO(g)+5H2O(g);ΔH=-1526kJ/
g)+9/2O2(g)=4CO(g)+5H2O(g);ΔH=-1526kJ/ mol
mol
由此判断,正丁烷的燃烧热是
| A.-2878kJ/mol | B.-2658kJ/mol | C.-1746kJ/mol | D.-1526kJ/mol |
 CuSO4(s)+5H2O(l) ΔH=+Q2 mol·L
CuSO4(s)+5H2O(l) ΔH=+Q2 mol·L 则Q1和Q2的关系为( )
则Q1和Q2的关系为( )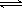 2NH3(g)ΔH=-92.4 kJ·mol
2NH3(g)ΔH=-92.4 kJ·mol ):
): 粤公网安备 44130202000953号
粤公网安备 44130202000953号