下列溶液中有关物质的量浓度关系正确的是
A.0.2 mol/L 的NaHCO3溶液: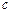 (HCO3_)> (HCO3_)>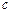 (CO32-)>0.1 mol/L > (CO32-)>0.1 mol/L >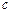 (H2CO3) (H2CO3) |
B.pH=2的CH3COOH溶液与pH=12的NaOH溶液等体积混合: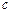 (Na+)> (Na+)>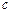 (CH3COO-)> (CH3COO-)>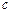 (OH-)> (OH-)>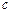 (H+) (H+) |
C.0.2 mol/L CH3COOH溶液和0.2 mol/L CH3COONa溶液等体积混合: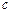 (CH3COO-)+ (CH3COO-)+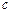 (OH-)- (OH-)-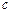 (H+)=0.1 mol/L (H+)=0.1 mol/L |
| D.0.1 mol/L Na2CO3溶液与0.1 mol/L NaHCO3溶液等体积混合: |
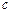 (CO32-)+2
(CO32-)+2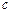 (OH-)=
(OH-)=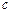 (HCO3_)+3
(HCO3_)+3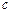 (H2CO3)+2
(H2CO3)+2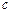 (H+)
(H+)
 A(g)+B(g),经一段时间后,甲、乙两容器反应都达到平衡。下列说法中正确的是( )
A(g)+B(g),经一段时间后,甲、乙两容器反应都达到平衡。下列说法中正确的是( ) xC(g) ΔH<0。测得两容器中c(A)随时间t的变化如图所示,下列说法正确的是 ( )
xC(g) ΔH<0。测得两容器中c(A)随时间t的变化如图所示,下列说法正确的是 ( )
 xC(g)达到平衡后,甲、乙两容器中A物质的浓度比为5:3,甲、乙两容器中C的体积分数大小为 ( )
xC(g)达到平衡后,甲、乙两容器中A物质的浓度比为5:3,甲、乙两容器中C的体积分数大小为 ( )
 2HI(g) ΔH<0,当达到平衡后,t1时刻改变反应的某一条件(混合气体总物质的量不变),造成容器内压强增大,则下列说法中正确的是 ( )
2HI(g) ΔH<0,当达到平衡后,t1时刻改变反应的某一条件(混合气体总物质的量不变),造成容器内压强增大,则下列说法中正确的是 ( )
 粤公网安备 44130202000953号
粤公网安备 44130202000953号