已知在25oC,101kPa下,1g C8H18(辛烷)燃烧生成二氧化碳和液态水时放出48.40kJ热量。表示上述反应的热化学方程式正确的是
A.C8H18(l)+ O2(g)= 8CO2(g)+9H2O(g);ΔH = -48.40kJ/mol O2(g)= 8CO2(g)+9H2O(g);ΔH = -48.40kJ/mol |
B.C8H18(l)+ O2(g)= 8CO2(g)+9H2O(l);ΔH = -5518kJ/mol O2(g)= 8CO2(g)+9H2O(l);ΔH = -5518kJ/mol |
C.C8H18(l)+ O2(g)= 8CO2(g)+9H2O(l);ΔH = +5518kJ/mol O2(g)= 8CO2(g)+9H2O(l);ΔH = +5518kJ/mol |
D.C8H18(l)+ O2(g)= 8CO2(g)+9H2O(l);ΔH = -48.40kJ/mol O2(g)= 8CO2(g)+9H2O(l);ΔH = -48.40kJ/mol |
 +H
+H
 H
H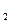 O+CO
O+CO +HCl溶液
+HCl溶液 ,该固体是()
,该固体是() O
O
 O
O
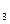 ·10H
·10H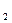 O,在空气里易风化
O,在空气里易风化 粤公网安备 44130202000953号
粤公网安备 44130202000953号